
Glyphosate is the most widely used herbicide worldwide. It is linked to cancer, liver and kidney disease, endocrine disruption, neurotoxicity and many other health concerns. First patented by the Monsanto Company, glyphosate is now manufactured and sold by many companies in hundreds of products. It is best known as the active ingredient in Roundup-branded herbicides, … Glyphosate: Cancer, liver disease, endocrine disruption and other health concerns

Residents of a North Carolina community – where the haze of pollution coats the inside and outside of residents’ homes with potentially harmful bacteria from nearby industrial animal farms – are working with researchers at Johns Hopkins University to test a technique that can measure pig manure in the dust.
Per- and Polyfluoroalkyl Substances (PFAS) are a group of thousands of manufactured chemicals widely used by a range of industries and commonly found in a large number of consumer products. One common characteristic of PFAS is that they persist in the environment and can accumulate in humans and animals. For this reason, they are often … PFAS ‘forever chemicals’ tied to cancer, birth defects

Long-term exposure to even small amounts of air pollution from vehicles promotes abnormal changes in the liver—potentially increasing the risk of fatty liver disease, according to new research in mice. Mice exposed to fine particulate matter (PM2.5, or tiny pollutant particles of 2.5 micrometers or less) for 12 weeks showed inflammation in the liver, more … Even low levels of traffic air pollution can damage the liver, new study shows

With legal and shareholder pressure mounting, Bayer AG on Thursday was preparing to launch an initiative to “regain public trust” after its acquisition last year of Monsanto Co. brought Bayer thousands of lawsuits filed by cancer victims and damning revelations of corporate deception surrounding years of health concerns about Monsanto’s glyphosate-based Roundup herbicides. The plan … Bayer Internal Emails Says Seeks to “Regain Public Trust” Amid Monsanto Mess

A senior scientist at the former Monsanto Co. on Tuesday told jurors in a California trial that regulators around the world support the company’s position that its glyphosate-based herbicides, such as the popular Roundup brand, are safe for users. Donna Farmer, who worked as a toxicologist at Monsanto for more than two decades and now … Monsanto scientist tells jurors company’s side of Roundup cancer controversy

Carey Gillam’s “Whitewash: The Story of a Weed Killer, Cancer and the Corruption of Science (Island Press) has received rave reviews since its release last fall and has received several awards for outstanding reporting: Winner, 2018 Rachel Carson Book Award from the Society of Environmental Journalists 2018 Gold Medal winner, Outstanding Book of the Year, … Rachel Carson Environment Book Award Winner: Whitewash by Carey Gillam

By Carey Gillam CAMBRIA, Calif.- Standing on the ridge overlooking her central California farm, new widow Teri McCall sees her husband Jack nearly everywhere. There, atop the highest hill, is where the couple married in 1975- two self-described “hippies’ who knew more about how to surf than farm. And over there, surrounded by the lemon, … What Killed Jack McCall? A Farmer Dies; A Case Against Monsanto Takes Root

USRTK Research Director Carey Gillam’s new book is out now and garnering glowing reviews. Here is a brief description of the book from publisher Island Press: Lee Johnson was a man with simple dreams. All he wanted was a steady job and a nice home for his wife and children, something better than the hard … The Monsanto Papers – Deadly Secrets, Corporate Corruption, and One Man’s Search for Justice

(Update with EPA explanation) In an unusual move, the Environmental Protection Agency (EPA) has deleted the name of a high-ranking U.S. health official from a public comment that warned of cancer links to the weed killing chemical glyphosate and called for a halt to industry manipulation of research. The public comment in question was submitted … EPA removes name of U.S. official from warning of glyphosate cancer links

By Carey Gillam This might have been a tough week for Monsanto Co. The Environmental Protection Agency was slated to hold four days of public meetings focused on essentially one question: Is glyphosate, the world’s most widely used herbicide and the lynchpin to Monsanto’s fortunes, as safe as Monsanto has spent 40 years telling us … EPA Bows to Chemical Industry in Delay of Glyphosate Cancer Review

As researchers we often look to documents to shed new light on issues important to food policy. Sometimes, they simply reflect what we already know. That’s the case with one new communication string that adds to evidence of a far-reaching strategy by food industry players to discredit and diminish the world’s leading cancer research agency. … Email of intrigue: “IARC is killing us!”

Carey Gillam is an investigative journalist and award-winning author who spent 17 years on the food and agriculture beat for Reuters. Gillam is now research director of the public interest research group U.S. Right to Know. These remarks were delivered Oct. 11, 2017 before a joint public hearing on “The Monsanto Papers and Glyphosate” before … Decades of Deceit: Carey Gillam’s presentation to the European Parliament glyphosate hearing

The mysterious delay of what was supposed to be a closely watched St. Louis showdown over claims that Monsanto’s Roundup herbicides cause cancer has stirred speculation that a settlement may be in the offing and heartened investors in Monsanto’s German owner Bayer, who feared a fourth trial loss. The trial in St. Louis, Monsanto’s former … Speculation Over Settlement as Roundup Cancer Trial Postponed

A federal judge on Monday had harsh words for Bayer AG’s plan to delay potential future Roundup cancer lawsuits and block jury trials, criticizing the highly unusual proposal crafted by Bayer and a small group of plaintiffs’ attorneys as potentially unconstitutional. The “Court is skeptical of the propriety and fairness of the proposed settlement, and … Court frowns on Bayer’s proposed Roundup class-action settlement

Insiders at the Environmental Protection Agency (EPA) have alleged dozens of violations of the agency’s “scientific integrity ” policy over the last few years, including complaints of political interference and tampering with chemical risk assessments, but nearly all the complaints have been ignored, according to an analysis conducted by a nonprofit group representing EPA employees. … EPA’s “scientific integrity” program lacks teeth, group alleges

Dicamba(3, 6-dichloro-2-methoxybenzoic acid) is a broad-spectrum herbicide first registered in 1967. The herbicide is used on agricultural crops, fallow land, pastures, turfgrass and rangeland. Dicamba is also registered for non-agricultural uses in residential areas and other sites, such as golf courses where it is primarily used to control broadleaf weeds such as dandelions, chickweed, clover … Dicamba: Concerns about health risks and crop damage

Concerns about the world’s most widely used herbicide are taking a new twist as researchers unveil data that indicates pervasive use of Monsanto Co.’s weed killer could be linked to pregnancy problems. Researchers looking at exposure to the herbicide known as glyphosate, the key ingredient in Monsanto’s Roundup branded herbicides, said they tested and tracked … Moms Exposed to Monsanto Weed Killer Means Bad Outcomes for Babies

By Carey Gillam The U.S. Department of Agriculture has quietly dropped a plan to start testing food for residues of glyphosate, the world’s most widely used weed killer and the key ingredient in Monsanto Co.’s branded Roundup herbicides. The agency spent the last year coordinating with the Environmental Protection Agency (EPA) and the Food and … USDA Drops Plan to Test for Monsanto Weed Killer in Food

The Canadian Food Inspection Agency has gone where the U.S. government dares not tread – testing thousands of foods commonly consumed by its citizens for residues of a controversial herbicide linked to cancer. And the findings are less than appetizing. The agency said it found the pesticide known as glyphosate, the key ingredient in Monsanto … Canadians Report Weed Killer Detected in 30 Percent of Food Tested

Also see: MDL Monsanto Glyphosate Cancer Case Key Documents and Analysisand US Right to Know Sues EPA for Release of Glyphosate Documents By Carey Gillam The puzzle pieces are starting to fall into place, but so far it’s not a pretty picture. A series of internal Monsanto Co. documents revealed this week via a court … Monsanto Weed Killer: Scientific Manipulation Revealed

By Carey Gillam Monsanto Co. and officials within the Environmental Protection Agency are fighting legal efforts aimed at exploring Monsanto’s influence over regulatory assessments of the key chemical in the company’s Roundup herbicide, new federal court filings show. The revelations are contained in a series of filings made within the last few days in the … Monsanto, EPA Seek to Keep Talks Secret On Glyphosate Cancer Review

The Food and Drug Administration (FDA) has resumed its first-ever endeavor to evaluate how much of a controversial chemical is making its way into the U.S. food supply. And the tests can’t come soon enough as safety concerns about the herbicide known as glyphosate grow. The FDA, the nation’s chief food safety regulator, launched what … FDA Resumes Testing Foods For Weed Killer, Safety Questions Grow

Newly released government email communications show a persistent effort by multiple officials within the Environmental Protection Agency (EPA) to slow a separate federal agency’s safety review of Monsanto’s top-selling herbicide. Notably, the records demonstrate that the EPA efforts came at the behest of Monsanto, and that EPA officials were helpful enough to keep the chemical … Collusion or Coincidence? Records Show EPA Efforts to Slow Herbicide Review Came in Coordination with Monsanto

Dozens of farmers around the United States are suing the former Monsanto Co., purchased in 2018 by Bayer AG, and conglomerate BASF in an effort to hold the companies accountable for millions of acres of crop damage the farmers claim is due to widespread illegal use of the weed killing chemical dicamba, use promoted by … The Dicamba Papers: Key documents and analysis
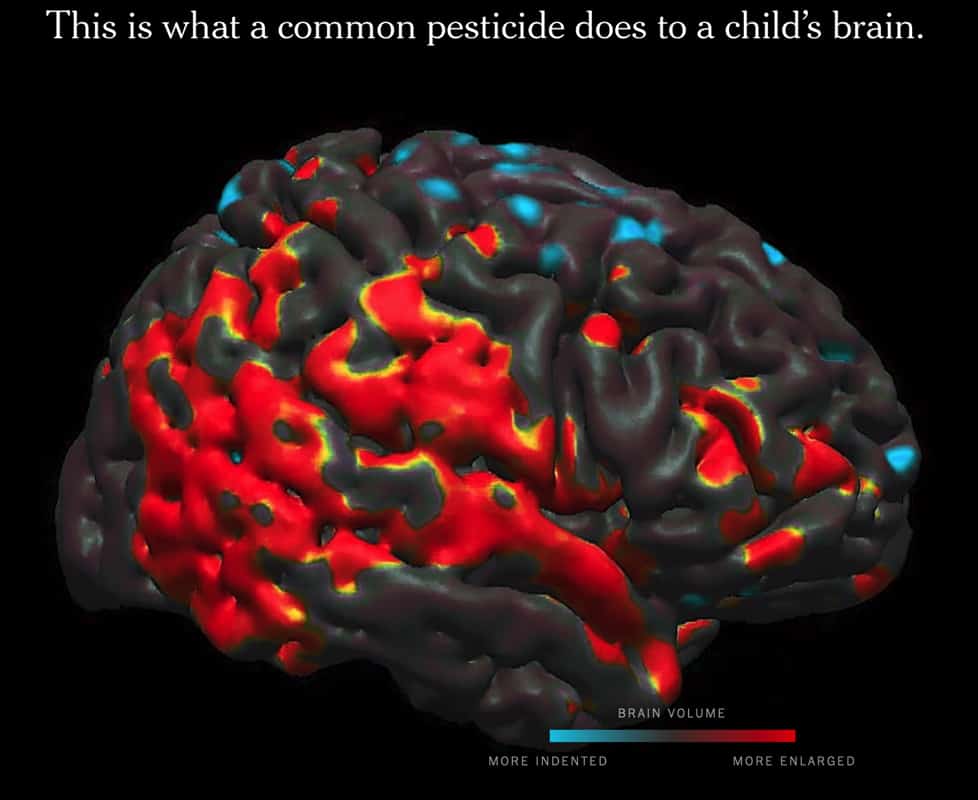
Scientific and medical studiesHistory and politics of chlorpyrifos Problems with industry studies Scientific research shows that chlorpyrifos, a widely used insecticide, is strongly linked to brain damage in children. These and other health concerns led several countries and some U.S. states to ban chlorpyrifos years ago, but the chemical was still allowed for use by … Chlorpyrifos: Pesticide tied to brain damage in children

A plan to delay any new Roundup cancer claims for years and shift the key question of whether or not the weed killer causes cancer from a jury to a hand-picked panel of scientists faces potential opposition from some of the plaintiffs’ attorneys who initiated and led the mass tort claims against Roundup maker Monsanto, … Challenge eyed to class action plan for Bayer Roundup settlement

By Carey Gillam Dewayne “Lee” Johnson has led what many might call an unremarkable life. The 46-year-old father and husband spent several years working as a school groundskeeper and spending free time teaching his two young sons to play football. But this week he takes center stage in a global debate over the safety of … Bay Area Man vs. Monsanto: First Trial Over Roundup Cancer Claims Set to Begin

When microbiologist Bruce Hemming was hired two years ago to test breast milk samples for residues of the key ingredient in the popular weed-killer Roundup, Hemming at first scoffed at the possibility.Hemming, the founder of St. Louis-based Microbe Inotech Laboratories, knew that the herbicidal ingredient called glyphosate was not supposed to accumulate in the human … USDA Shirking Obligation to Give Consumers Clarity Over Herbicide Residues on Food

Lawyers involved in U.S. litigation accusing Syngenta AG of spending decades selling an herbicide that causes Parkinson’s disease are moving toward selection of a bellwether trial to be held roughly a year from now, according to the federal judge overseeing the litigation. In a hearing on Friday, U.S. District Judge Nancy Rosenstengel of the Southern … Litigation alleging Syngenta’s paraquat weed killer causes Parkinson’s disease moving toward trial

A federal judge has denied Swiss chemical company Syngenta’s effort to throw out one of a growing number of lawsuits alleging the company’s weed killing products cause Parkinson’s Disease. The decision offers a boost for the expanding number of law firms and plaintiffs making similar claims. In an April 12 ruling, U.S. District Judge John … Federal court rejects Syngenta’s bid to toss lawsuit over paraquat herbicide

Six more lawsuits alleging Syngenta’s weed killing pesticide paraquat causes Parkinson’s Disease were filed last week in Pennsylvania, California and Illinois, adding to more than a dozen similar lawsuits already filed in U.S. courts. The lawsuits all allege that exposure to paraquat, which is banned in more than 30 countries though not in the … Paraquat litigation grows, first trial set for May 10

A highly anticipated first-ever trial pitting a group of farmers against the global agricultural giant Syngenta AG over allegations that Syngenta’s paraquat weed killer causes Parkinson’s disease has been delayed until June, the parties involved said on Saturday. The trial was set to begin Monday, livestreamed by Courtroom View Network, but a continuance was ordered … Trial pitting farmers against Syngenta delayed until June

(UPDATED May 18 with pretrial order) As Bayer AG works to put an end to costly litigation over alleged connections between Roundup herbicide and cancer, the company faces a critical hearing on Wednesday in federal court in San Francisco. At issue in the hearing is a proposed $2 billion class action settlement structured by Bayer … Key court hearing Wednesday in Bayer cancer liability litigation

A highly anticipated first-ever trial pitting a group of farmers against the global agricultural giant Syngenta AG over allegations that Syngenta’s paraquat weed killer causes Parkinson’s disease has been delayed again and may not take place at all, according to sources close to the case. The trial in the case of Hoffman V. Syngenta was … Another delay for trial set to examine allegation that Syngenta weed killer causes Parkinson’s

See Carey Gillam’s article in The Guardian, Corporate studies asserting herbicide safety show many flaws, new analysis finds (July 2, 2021). In this post we provide links to the 53 once-secret studies and related materials. Questions about the safety of glyphosate-based herbicides (GBHs) have persisted for years, as scientific research has split over whether or … New analysis of glyphosate industry studies finds them outdated, flawed

Children’s health expert Ruth Etzel on Monday testified in a public hearing that the Environmental Protection Agency (EPA) worked to silence and harass her, removing her from a top-level position after she complained about agency delays in advancing a lead poisoning prevention program. In testimony before Merit Systems Protection Board Judge Joel Alexander, Etzel described … EPA whistleblower testifies her advocacy for stronger health protections drew agency retaliation

Syngenta AG is facing a growing number of U.S. lawsuits over allegations that its paraquat herbicide causes Parkinson’s disease, with a Fresno, California man pushing for an expedited trial that potentially would start within the next few months, and multiple plaintiffs’ lawyers jockeying for power and influence over future trial proceedings. Plaintiff George Isaak used … Litigation against Syngenta grows; lawyers fight over evidence and trial dates

The U.S. Environmental Protection Agency (EPA) has a long and well-documented history of questionable conduct when it comes to regulation of chemicals important to the profit centers for many large and powerful corporations. Numerous examples show a pattern of agency actions that allow for the use of dangerous chemicals by consumers, farmers, groundskeepers and others … EPA exposed for hiding chemical risks, favoring corporate interests

Multiple lawsuits are pending in the United States against Syngenta alleging the weedkilling chemical paraquat causes Parkinson’s disease. A notice of settlement was filed June 18, 2021 for several paraquat cases. See this document. But more than 400 lawsuits are pending. The lawsuits name Syngenta as well as Chevron Phillips Chemical Co. and Growmark Inc. … Paraquat Papers – Updates to U.S. litigation

After two costly courtroom losses, lawyers for Monsanto and its German owner Bayer AG on Wednesday were set to make closing arguments in what is the third trial brought by people who blame their cancers on use of Monsanto’s Roundup and other glyphosate-based weed killer brands. Plaintiffs Alva and Alberta Pilliod, a married couple in … Sparks to Fly in Closing Arguments at Third Roundup Cancer Trial

ACalifornia appeals court should reject efforts by Monsanto to overturn a jury verdict awarding millions of dollars to a school groundskeeper and approve $250 million in punitive damages the jury ordered a year ago this month in the first Roundup cancer trial, according to a brief in the case filed Monday. The brief filed by … “Serious, Deadly Injury” Cited in New Appeals Court Filing Over Roundup Cancer Claims

Thisarticlewas originally published in the San Francisco Chronicle. By Nathan Donley and Carey Gillam It’s been three weeks since a San Francisco jury found that exposure to Monsanto’s Roundup herbicides contributed to former school groundskeeper Dewayne “Lee” Johnson’s terminal cancer and awarded a stunning $289 million in damages to the 46-year-old father. And during that … SF Roundup Case Demonstrates Importance of Independence in Scientific Evidence

After several months out of the headlines, lawyers for both sides of the nationwide Roundup cancer litigation are gearing up for overlapping trials in the new year as several more cancer patients seek to blame Monsanto for their diseases. Six trials are currently set to take place starting in January, with one in February, two … Six Monsanto Roundup Cancer Trials Set for January