Key scientific studies
History of deceptive marketing
Sucralose is the most widely used artificial sweetener in the United States. Most commonly sold under the brand name Splenda, sucralose is used in over 6,000 food products. It is often found in “diet” sodas including Diet Coke with Splenda, Diet Pepsi with Splenda, as well as Gatorade’s Propel Water, low-calorie Kool-Aid, Atkins Diet products, and other low-calorie foods and drinks.
Sucralose is 600 times sweeter than table sugar and itself contains no calories. Although it has been marketed as a healthy product that can help fend off obesity and diabetes, studies suggest that sucralose consumption is linked to leukemia, weight gain, obesity, diabetes, liver inflammation, metabolic dysfunction and other illnesses.
Sucralose backers have also claimed it is poorly absorbed and does not significantly bioaccumulate in the human body. However, a 2018 study found that sucralose metabolizes and bioaccumulates in rats. Based on this recent science, U.S. Right to Know petitioned the Federal Trade Commission to investigate deceptive advertising claims by Tate & Lyle and Coca-Cola.

Key facts about sucralose risks
In May 2023, the World Health Organization advised people not to consume non-sugar sweeteners, including sucralose, for weight loss. The recommendation is based on a systematic review of the most current scientific evidence that links non-sugar sweeteners to type 2 diabetes, cardiovascular diseases and all-cause mortality, as well as increased body weight.
The Food and Drug Administration approved sucralose for use in 1998 in 15 food categories, and as a general purpose sweetener a year later. It was the fastest shift in FDA’s history from a specified usage to general purpose approval of an artificial sweetener.
Of the over 100 sucralose studies FDA reviewed at the time, none involved humans, only three lasted more than a year, and many of them were not even published for public scrutiny.
Subsequent studies, including longitudinal ones involving human populations, have linked sucralose to a range of health dangers. FDA has not reevaluated its authorization with the current science.
FDA’s 1998 authorization claims that “sucralose is relatively poorly absorbed” into the body. Recent science casts doubt on that claim (see USRTK petition to FTC).
In a 2008 interview, former FDA official Alan Rulis discussed “rocky moments” in the sucralose petition process: “We discovered, way too late in the process, I think, that there was an unresolved issue that had to do with the test animals in some studies showing a more-than expected body-weight-gain decrement while on sucralose dosing.” The company provided data to resolve FDA’s concerns, Rulis said.
Food Chemical News reported that, in 1995, McNeil Nutritionals — a Johnson & Johnson subsidiary and marketer of Splenda — had planned to submit its product approval application; but, “in the process of completing a six-month clinical study in diabetic patients…[t]hat study raised concerns about the effect of sucralose on blood sugar in those individuals, and McNeil asked the agency to withhold its final decision until additional work could be done.”
What is sucralose?
Sucralose (known as E 955 in the European Union) is synthesized by chlorinating the sugar sucrose, by substituting three hydroxyl groups with chlorine atoms. Its chemical structure can be seen below.
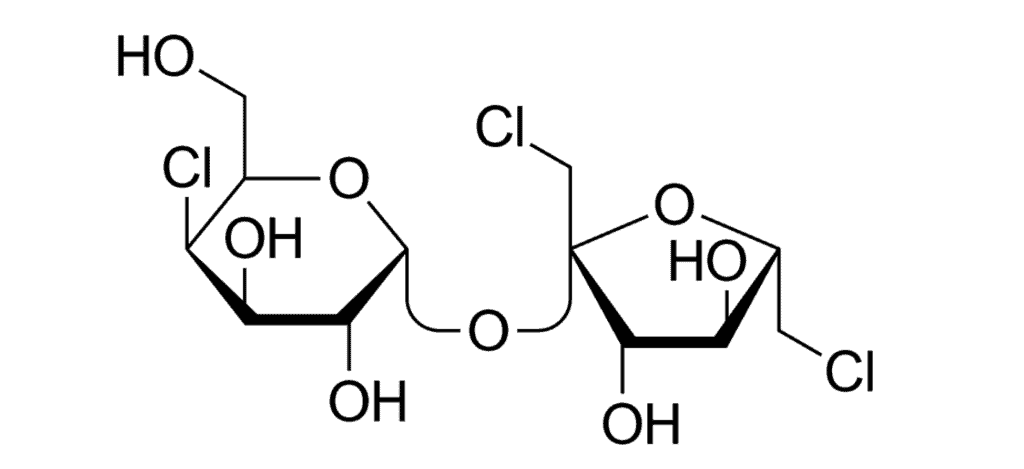
Sucralose was invented by accident in 1975 when a laboratory leader at Queen Elizabeth College told an assistant to “test” the chemical, which he understood at the time as “taste.” After discovering the sweet taste of the compound originally under consideration as an insecticide, the team continued its scientific work. The research team filed for a patent in 1976, and received it in 1984.
“Sucralose” is a marketing name Tate & Lyle invented, with no science-based etymology. The compound’s proper chemical name is trichlorogalatosucrose. Because the word sucralose is similar to sucrose (a naturally occurring sugar) it falsely expresses an easy similarity with a natural sugar.
In its first decade on the market, McNeil Nutritionals (then a subsidiary of Johnson & Johnson) marketed Splenda as “made from sugar, so it tastes like sugar.” Several regulatory agencies deemed this to be deceptive advertising.
Sucralose is most commonly sold as Splenda. Other brand names include Cukren, Zerocal, Nevella, Candys, Sukrana, Canderel Yellow and SucraPlus.
What are the health risks of sucralose?
Scientific studies of sucralose reveal the following health risks:
Leukemia
A 2016 study from researchers at the Ramazzini Institute published in the International Journal of Occupational and Environmental Health examined sucralose consumption in mice. Researchers found “a significant dose-related increased incidence of males bearing malignant tumors and a significant dose-related increased incidence of hematopoietic neoplasias in males,” particularly at doses of 2,000 and 16,000 parts per million. “These findings do not support previous data that sucralose is biologically inert,” the authors concluded. “More studies are necessary to show the safety of sucralose … Considering that millions of people are likely exposed, follow-up studies are urgent.”
Obesity, diabetes, weight gain, increased appetite, metabolic dysfunction
A March 2025 randomized crossover trial in Nature Metabolism studied the effects of sucralose, sucrose, and water on brain activity and hunger in 75 young adults. The results showed that sucralose increased hypothalamic blood flow and hunger responses compared to sucrose, which raised blood sugar and reduced hypothalamic activity. The findings suggest that non-caloric sweeteners may influence brain mechanisms that regulate appetite.
- This Common Artificial Sweetener Could Be Making You Hungrier, Parade Magazine (3.26.25)
A July 2023 analysis in Diabetes Care of 105,588 participants from the French NutriNet-Santé study finds in a 9.1 year follow-up that, compared with non-consumers, higher consumers of artificial sweeteners had higher risks of developing type-2 diabetes. Positive associations were also observed for individual artificial sweeteners aspartame, acesulfame-K and sucralose.
A February 2023 sucralose study in Biomedicines reports that newborns from mothers who intensely consumed sucralose during pregnancy were heavier and exhibited markers of metabolic alteration and low-grade systemic inflammation. “Robust data in animals show that sucralose intake during gestation can predispose the offspring to weight gain, metabolic disturbances, and low-grade systemic inflammation, however, concluding information remains elusive in humans,” the researchers note. In this cross-sectional prospective study, researchers compared the birth weight of infants born to mothers with light (LSI) and heavy sucralose intake (HSI). Newborns born to mothers with HSI “displayed significant increases in birth weight and insulin compared to newborns from LSI mothers … (and) showed a substantial increase in the percentage of inflammatory nonclassical monocytes compared to neonates from LSI mothers.” The results “demonstrate that heavy sucralose ingestion during pregnancy affects neonates’ anthropometric, metabolic, and inflammatory features.”
A 2014 study in Nature pointed to risks of consuming artificial sweeteners generally, and sucralose specifically, for diabetes patients — a core market for sucralose. The paper concluded that consumption of artificial sweeteners “drives the development of glucose intolerance through induction of compositional and functional alterations to the intestinal microbiota.” The increase in artificial sweetener consumption, the study notes, “coincides with the dramatic increase in the obesity and diabetes epidemics. Our findings suggest that [artificial sweeteners] may have directly contributed to enhancing the exact epidemic that they themselves were intended to fight.”
A 2013 review article published in Trends in Endocrinology & Metabolism concludes that sucralose and other artificial sweeteners may cause weight gain and negatively affect gut health. The paper discusses accumulating evidence that consumers of sugar substitutes may be at increased risk of excessive weight gain, metabolic syndrome, type 2 diabetes, and cardiovascular disease. The paper posits that “consuming sweet-tasting but noncaloric or reduced-calorie food and beverages interferes with learned responses that normally contribute to glucose and energy homeostasis. Because of this interference, frequent consumption of high-intensity sweeteners may have the counterintuitive effect of inducing metabolic derangements.”
- Professor: Diet drinks are not the sweet solution to fight obesity, health problems, Purdue University (7.11.13)
A 2013 study published in Diabetes Care found that “the ingestion of sucralose alters the metabolic response to an oral glucose load in obese people who are not regular consumers” of the substance. “These findings support the notion that sucralose is not metabolically inert but has physiologic effects.”
A 2016 study published in Cell Metabolism found that “chronic consumption of [sucralose] triggers a conserved neuronal fasting response and increases the motivation to eat.” After chronic exposure to sucralose, “we saw that animals began eating a lot more,” a co-author of the study explained in a press release. “Through systematic investigation of this effect, we found that inside the brain’s reward centres, sweet sensation is integrated with energy content. When sweetness versus energy is out of balance for a period of time, the brain recalibrates and increases total calories consumed.”
- Why artificial sweeteners can increase appetite … A comprehensive new study by Sydney researchers has revealed for the first time why this response occurs, Garvan Institute of Medical Research news release (7.13.16)
A 2008 study in the Journal of Toxicology and Environmental Health, Part A by Duke University researchers found that rats exposed to Splenda at below, equal to, and above FDA-sanctioned median Acceptable Daily Intake levels, for 12 weeks showed “numerous adverse effects,” including reduced beneficial fecal microflora, increased fecal pH and enhanced expression levels of proteins known to limit the bioavailability of orally administered drugs and nutrients. The rats also experienced weight gain even at consumption levels below the FDA’s recommended Acceptable Daily Intake advisory.
Insulin impacts
A 2020 study by Yale researchers in Cell Metabolism found that “consuming seven sucralose-sweetened beverages with, but not without, a carbohydrate over 10 days decreases insulin sensitivity in healthy human participants.” The findings imply that “(1) carbohydrate metabolism is altered in the presence of … sucralose and (2) that this alteration leads to decreases in peripheral and central sensitivity to sugar and sweet taste.” Of particular concern, the authors note, “the metabolic changes we observed followed a very limited exposure.” These findings “raise the possibility that the combination effect may be a major contributor to the rise in the incidence of type 2 diabetes and obesity. If so, the addition of [low calorie sweeteners] to increase the sweetness of carbohydrate-containing foods should be discouraged and consumption of diet drinks with meals should be counseled against.”
A 2018 study in the American Journal of Clinical Nutrition concluded that research subjects who consumed sucralose “showed a significant decrease in insulin sensitivity,” leading the researchers to conclude: “Sucralose may have effects on glucose metabolism.” Lowered insulin sensitivity, sometimes called insulin resistance, can lead to higher blood sugar levels and the development of type 2 diabetes. “Our study provides confirmatory evidence that sucralose has a negative impact on insulin action, even in healthy individuals,” the researchers concluded.
A 2018 study in Nutrition pointed to impacts on insulin secretion and by extension, risk of type 2 diabetes, among healthy sucralose consuming subjects. “Long-term consumption of sucralose can develop insulin resistance and decrease AIR [acute insulin response], which may represent the earliest sign of development of type 2 diabetes mellitus,” researchers wrote. “Our study also demonstrated reduced AIR after a 4-wk ingestion of sucralose. This result may imply that chronic exposure to sucralose leads firstly to increased insulin secretion, and later to reduction of insulin secretion.”
A 2019 study in the Journal of Immunology Research found that “a 48 mg sucralose sip increases serum insulin and unbalances monocyte subpopulation…in noninsulin-resistant healthy young adults.” Heightened insulin levels, known as hyperinsulinemia, increases the risk of obesity, type 2 diabetes, cardiovascular disease, and cancer. The authors wrote, “The apparently innocuous consumption of sucralose should be reexamined in light of these results.”
In 2022, a 10-week human study of sucralose consumption published in Microorganisms, concluded that “sucralose amounts, far lower than the suggested [acceptable daily intake], alter the balance of the gut microbiome, while also being associated with significant elevations in [glucose levels] and serum insulin in response to glucose loads.”
Genotoxicity
A May 2023 study in the Journal of Toxicology and Environmental Health examined the toxicological and pharmacokinetic properties of sucralose-6-acetate, an impurity in the manufacture of sucralose that is found in products containing sucralose: “findings for sucralose-6-acetate raise significant health concerns regarding the safety and regulatory status of sucralose itself,” the researchers report. The study also establishes that sucralose-6-acetate is genotoxic, meaning it can damage DNA.
- Chemical Found in Common Sweetener Damages DNA, North Carolina State University news release (5.31.23)
Testicular damage and male infertility
A May 2025 mouse study in Environmental Health Perspectives provides “new insights into the adverse effects of sucralose on male reproductive physiology, highlighting its role in disrupting autophagy, inducing oxidative stress, and impairing reproductive function.” Cells exposed to sucralose via in vitro experiments “had significantly lower cell survival rates. Sucralose exposure significantly reduced cell viability in TM3 and TM4 cells, induced oxidative stress, and disrupted autophagic flux by impairing autophagosome–lysosome fusion. Additionally, sucralose downregulated T1R3 protein expression, suggesting a role for sweet taste receptor signaling in testicular cell regulation. In vivo, chronic oral exposure to sucralose led to decreased sperm viability and dysregulated reproductive function, including altered testicular morphology and suppressed steroidogenesis.”
Sucralose in human breast milk and babies
Sucralose can enter into the breastmilk of breastfeeding mothers, according to a 2019 study in the Journal of Pediatric Gastroenterology and Nutrition. The study of 34 breastfeeding women concludes that “sucralose appeared in breast milk two hours following ingestion, with concentrations varying markedly between individuals.” Since the study assessed breast milk after just a single diet soda ingestion, researchers note that concentrations reported “may underestimate true infant exposure via the breast milk.” Future research should determine concentration after repeated exposures, and whether chronic ingestion of artificial sweeteners via breast milk has clinically relevant health consequences including “alteration of taste preferences, gut microbiota, metabolism and weight trajectory” of infants.
A 2020 study published in Gut Microbes concluded that sucralose consumption in pregnant mice “inhibited intestinal development, induced imbalance of gut microbiota and low-grade inflammation, and further disrupted gut barrier function in [three-week-old] offspring.” The researchers wrote, “These data suggest that excessive sucralose should be taken with caution especially during pregnancy and lactation” and also provide “new insight into a better understanding of the pathogenesis of [nonalcoholic fatty liver disease] in adulthood.”
Bioaccumulation
A 2018 study published in the Journal of Toxicology and Environmental Health concluded that sucralose could be seen in rat tissue “two weeks after cessation of the 40-day feeding period even though this compound had disappeared from the urine and feces.” These findings “do not support the claims previously submitted to regulatory agencies that sucralose is a stable compound that (1) is not metabolized in vivo, (2) excreted unchanged in the feces, and (3) clears the body within a few days,” concluded the researchers. “Data indicate that it may now be time to revisit the regulatory status of sucralose.”
- Study Finds Sucralose Produces Previously Unidentified Metabolites, North Carolina State University news release (8.27.18)
Formation of toxic or carcinogenic compounds
Sucralose dangers may also include toxic compounds released when the sweetener is heated, according to these studies:
In a 2019 study, the German Federal Institute for Risk Assessment (BfR) pointed to cancer risks associated with cooking foods containing sucralose at a temperature of 250°F or higher. Cooking at this temperature “may lead to the formation of chlorinated organic compounds with a health-damaging potential, such as polychlorinated dibenzo-p-dioxins (PCDD), dibenzofurans (PCDF) and chloropropanols,” the study concludes. Consumption of these hazardous substances and toxins could lead to diseases such as cancer, the skin disorder chloracne, as well as liver and kidney damage.
- Harmful compounds might be formed when foods containing the sweetener sucralose are heated, BfR news release (4.9.19)
In 2020, the German agency BfR published a review of 19 studies on sucralose in the journal Food Chemistry. The researchers concluded that “sucralose can be degraded at high temperatures, e.g. during cooking or baking of sucralose-containing foods,” and that, “As a consequence potentially toxic chlorinated compounds might be generated.”
A 2015 study published in Scientific Reports found, “decomposition in form of CO2 along with the formation of hydrogen chloride and other minor compounds” in heated food substances containing sucralose at temperatures of 200°F and above. The study concluded, “These findings not only corroborate the suspected instability of sucralose to high temperatures, but also indicate that even exposed to mild conditions the formation of hazardous polychlorinated compounds is observed.”
Irritable bowel syndrome
In a 2018 study published in Inflammatory Bowel Diseases, researchers found that, given over a six-week period, the artificial sweetener sucralose worsens gut inflammation in mice with Crohn’s disease. It had no substantive effect on those without the condition. “Our findings suggest that patients with Crohn’s disease should think carefully about consuming Splenda or similar products containing sucralose and maltodextrin,” the study’s lead author said in a press release.
- Artificial sweetener could intensify symptoms in those with Crohn’s disease. Promotes ‘bad’ bacteria and intestinal inflammation; findings may guide dietary habits in human patients, Case Western Reserve University School of Medicine (3.15.18)
Colon cancer
A 2020 article in Frontiers in Oncology based on research on mice, raised concerns about colon cancer risks associated with sucralose consumption. The study concluded that “sucralose caused significant increases in the number and size of [cancerous colon] tumors. A likely mechanistic explanation would be that inflammation was exacerbated by sucralose.” The study further surmised that a steady stream of sucralose in the diet could lead to “impaired inactivation of digestive protease, damage to the gut barrier, and exacerbated inflammation.”
Liver inflammation
A 2017 study of mice in Frontiers in Physiology reported, “Sucralose consumption for 6 months altered the gut microbiome composition, fecal metabolites, and pro-inflammatory gene expression in the liver. The alterations induced by sucralose consumption could affect the development of inflammation and further influence other physiological functions in the body.”
Can sucralose contribute to severe SARS-Cov2?
In a May 2023 letter to the BMJ Open Gastroenterology, scientists from North Carolina State University at Raleigh noted recent research that reported a significant decrement in gut bifidobacteria in persons with severe Covid. “This caught our attention because the common artificial sweetener sucralose (eg, Splenda) has a profound effect on the microbiome including significant reductions in bifidobacteria in the gut,” they wrote. “This raises the question of whether the organochlorine sweetener sucralose, which is present in tens of thousands of food products, may contribute to the observed depletion in bifidobacteria and low bacterial diversity found in severe SARS-CoV-2.” Additional research is needed, they said.
Review studies on non-sugar sweeteners
Two review studies published in 2023 raised health concerns about the class of non-sugar sweeteners, which include acesulfame K, aspartame, advantame, cyclamates, neotame, saccharin, sucralose and stevia.
In May 2023, the World Health Organization advised people not to consume non-sugar sweeteners, including sucralose. The recommendation is based on the agency’s systematic review of the most current scientific evidence that consumption of non-sugar sweeteners is associated with increased risk of type 2 diabetes, cardiovascular diseases and all-cause mortality, as well as weight gain.
- “Replacing free sugars with NSS does not help with weight control in the long term. People need to consider other ways to reduce free sugars intake, such as consuming food with naturally occurring sugars, like fruit, or unsweetened food and beverages,” Francesco Branca, WHO Director for Nutrition and Food Safety, said in a WHO news release. “People should reduce the sweetness of the diet altogether, starting early in life, to improve their health.”
A separate review published in May 2023 in Advances in Nutrition includes data of 11 meta-analyses obtained from 7 systematic reviews (51 cohort studies and 4 case-control studies). The review reports that artificially sweetened beverages are “associated with higher risk of obesity, type II diabetes, all-cause mortality, hypertension, and cardiovascular disease incidence (supported by highly suggestive evidence).”
Splenda’s deceptive advertising
Splenda has a long history of deceptive advertising. In its early years, McNeil Nutritionals (a subsidiary of Johnson & Johnson) spent over $200 million marketing Splenda with the misleading slogan “made from sugar so it tastes like sugar,” even though the product contains no natural sugars. The advertising campaign was the target of multiple lawsuits and regulatory reviews across the world. Courts or agencies in France, Australia, and New Zealand ruled the slogan was false and misleading, and banned the advertisements from their respective countries.
- The Australia Advertising Claims Board ruled in 2006 that for the “made from sugar so it tastes like sugar” slogan “reasonable members of the public viewing the Advertisement are likely to conclude that, at the very least, a significant proportion of the SPLENDA® product is made from some modified form of sugar.” The Board ruled that the ad “is likely to mislead or deceive viewers” and ordered it discontinued.
- New Zealand’s Advertising Standards Complaints Board refused the same advertisement in a 2005 ruling, on the grounds that it “gave rise to a likelihood of a consumer being confused and misled as a result of the comparison in the advertisement.”
- In 2007, the Commercial Court of Paris ruled that McNeil violated French consumer protection laws and ordered the company to stop what it concluded was misleading wording in its advertising. The court also ordered McNeil to pay 40,000 Euros in damages to Merisant, which manufactures artificial sweeteners made with aspartame (Equal and NutraSweet).

In the U.S, a similar lawsuit between Merisant and McNeil concluded with an undisclosed settlement. Splenda’s current marketing in the U.S. no longer focuses on sugar, but on claiming health benefits for diabetes patients and people who are trying to lose weight – despite science linking sucralose to obesity, diabetes, weight gain, increased appetite and metabolic dysfunction.
In recent years, as evidence has grown that sucralose may be bad for you, Splenda has launched advertising campaigns that focus on “debunking” what manufacturers call “junk science” that raises health concerns about sucralose or its side effects. Marketing campaigns, such as this read between the headlines contest, target dieticians, nutritionists, doctors and nurses asking them to refute the “myths” about health concerns linked to Splenda. The program was run by Ketchum public relations firm, which also has a long history of using deceptive tactics.
Splenda also partners with “science communicators” to downplay sucralose health risk, such as Yvette d’Entremont, aka “SciBabe,” who promotes diet soda and claims to correct misinformation about artificial sweeteners and pesticide products. However, she did not always disclose that she had been paid by Splenda and other companies to promote their products.
Journalism, opinion, and other studies
World Health Organization Warns Against Using Artificial Sweeteners: Continued consumption doesn’t reduce weight and could increase the risk of Type 2 diabetes, cardiovascular diseases and mortality in adults, the W.H.O. said, The New York Times (5.15.2023).
Sucralose might be making you fatter and sicker, The Washington Post (3.10.20)
Relationship between Research Outcomes and Risk of Bias, Study Sponsorship, and Author Financial Conflicts of Interest in Reviews of the Effects of Artificially Sweetened Beverages on Weight Outcomes: A Systematic Review of Reviews, by Daniele Mandrioli , Cristin E Kearns, and Lisa A. Bero. PLOS ONE (9.8.2016)
Killing Us Sweetly: How to Take Industry out of the FDA by Jason Iuliano, Journal of Food Law & Policy (2010).
Life after aspartame, by Pat Thomas, The Ecologist (8.9.2005)
What made Canada become a country with the highest incidence of inflammatory bowel disease: Could sucralose be the culprit? by Xiaofa Qin, Canadian Journal of Gastroenterology and Hepatology (9.9.2011).
Sucralose, A Synthetic Organochlorine Sweetener: Overview Of Biological Issues, by Susan S. Schiffman & Kristina I. Rother, Journal of Toxicology and Environmental Health (11.12.2013).
The not-so-sweet effects of sucralose on blood sugar control, by M. Yanina Pepino, American Journal of Clinical Nutrition (9.1.2018).
Sucralose revisited: Rebuttal of two papers about Splenda safety, by Susan S. Schiffman and Mohamed B.Abou-Donia, Regulatory Toxicology and Pharmacology (8.2012).
Researchers uncover how sugar substitutes disrupt liver detoxification, by Experimental Biology (4.5.2022).









